As this will be a physics/chemistry lesson, I shall try to make it as simple as possible for those who have not had grounding in the sciences. Everyone knows that water is H2O, and that the symbol stands for 2 parts Hydrogen and 1 part Oxygen. That is a good starting point.
Matter consists of Atoms and Molecules. Atoms are the basic building blocks of the Universe, the smallest being Hydrogen and the most complex being Uranium. Atoms combine to form Molecules. We say that water is a Molecule because it consists of three atoms combined together, two of Hydrogen and one of Oxygen.
Atoms consist of two components, the Nucleus and Orbiting Electrons. It is rather like the Solar System, with the Sun as the Nucleus, and Planets the Orbiting Electrons. The Nucleus contains Protons (positively charged) and Neutrons (without electric charge.) Hydrogen consists of just one Proton and one Orbiting Electron. Uranium consists of 92 Protons, 146 Neutrons, and 92 Orbiting Electrons.
Electrons occupy distinct Orbits round the Nuclei according to the Quantum Theory. They are grouped in Shells. The first shell, called the K shell can only contain 2 electrons. The L shell has a maximum of 8 electrons, the M shell a maximum of 18, and so on. Think again of the Solar System. The K shell is like the innermost planet Mercury, the L shell like Venus, and the M shell like Earth. Most of the matter of atoms is found in the Nucleus; the rest is just space, as it is between the planets.
We are interested in the two elements forming water, namely Oxygen and Hydrogen. Hydrogen has just one electron in the K shell, Oxygen, the 8th element, has 2 electrons in the K shell and 6 in the L shell. When these three elements combine to form water the electrons are shared between the elements, making more complicated orbits. We refer to these connections as Covalent Bonds. The electrons of the two Hydrogen atoms attach themselves to two of the six electrons in the L shell of Oxygen, leaving two pairs of unattached electrons in the L shell.
We now have to introduce what is called the Electronegativity of the elements, given the symbol X. Because atoms are composed of electrically charged units, they exhibit attractions depending on their structure. And because the maximum effect results from the outermost orbits of electrons, which are negatively charged, they tend to exert attractions upon other nearby atoms and molecules.Hence the term Electronegativity. The element with the strongest value of X is Fluorine, where X = 4. Our interest in this stems from the relative strengths of X in forming molecules.
The value of X for Oxygen is 3.5, and that of Hydrogen is 2.1. We are now going to introduce one other element, Carbon, with X = 2.5, so that we can compare two molecules, Water and Carbon Dioxide, both of which contain just three Atoms.
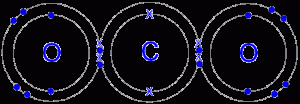
There is a similar sharing of electrons in Carbon Dioxide to that of Water, and it makes an ideal contribution to see how theelectronegativities operate. We may write the CO2 molecule as O=C=O and the H2O molecule as H=O=H. The difference in values of X for Oxygen and Carbon is 3.5 – 2.5 = 1.0, but for Oxygen and Hydrogen it is 3.5 – 2.1 = 1.4. The smaller the difference in X, the more straightforward is the bond that holds them together, so that we find CO2 Molecules more strongly bonded than H2O Molecules.
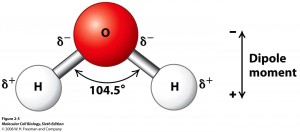
It is found that in the Carbon Dioxide molecule, the bonding is accurately described by the symbol O=C=O, but for water this linear arrangement is displaced due to the larger difference in X. A more accurate diagram for the structure of the water molecule would therefore be
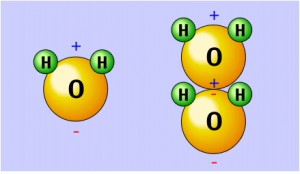
This lack of symmetry is the reason why water is so different to carbon dioxide, and why all the amazing properties of water exist. The two hydrogen atoms are separated by an angle of 104.5 degrees, making the molecule slightly Polar, that is to say, it behaves rather like a magnet with two opposite poles, in this case two electric poles. The Oxygen Atom is slightly negative, and the Hydrogen Atoms are slightly positive, causing the molecule to attract others. Water Molecules bond together in the frozen state in a hexagonal arrangement as a result. Hence Water is a Polar Molecule, whereas Carbon Dioxide is a Linear Molecule.
The Lord designed the Water Molecule with its Bent structure so that it would behave very differently to Carbon Dioxide, which is a gas at normal temperatures, but sublimates into “dry ice” at -78 degrees C. If Water Molecules arranged themselves linearly, water would boil at -100 degrees C, instead of +100 C, and we should never know water. It would just be a gas like oxygen, nitrogen, and hydrogen. This difference has come about because of the difference in the values of electronegativity. Who made it thus? If God had given Hydrogen the same value of X as Carbon, our world would be a very different place, and life as we know it could never exist.
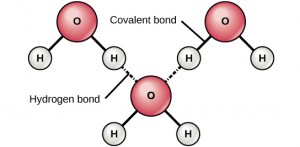
When water molecules align with each other, a weak bond is established between the negatively charged Oxygen atom of one water molecule and the positively charged Hydrogen atoms of a neighbouring water molecule, known as a Hydrogen Bond. Hydrogen bonds are very common in living organisms; for example, hydrogen bonds form between the bases of DNA to help hold the DNA chain together. Hydrogen bonds give water molecules two additional characteristics: cohesion and surface tension.
Because of the extensive hydrogen bonding in water, the molecules tend to stick to each other in a regular pattern. This phenomenon, called Cohesion, allows tall trees to bring water to their highest leaves from sources below ground.
A special type of cohesion is Surface Tension. The tension on the surface of water occurs when water molecules on the outside of the system align and are held together by Hydrogen Bonding to create an effect similar to a net made of atoms. For example, the surface tension of water allows water spiders to literally walk on water. Raindrops form into perfect spheres, without which we would never see a rainbow.
Were it not for Water being a Bent, Polar Molecule none of these things could happen.
I hope the above notes have made sense. As a scientist I have been privileged to see into the wonders of life at the atomic level, and marvel at the way in which God has put things together. There is no room here for evolution. There is far too much evidence of design, too wonderful for most to comprehend. Let me know if I have succeeded in helping you understand and appreciate these wonders.